No products in the cart.
Description
PRODUCT DESCRIPTION:
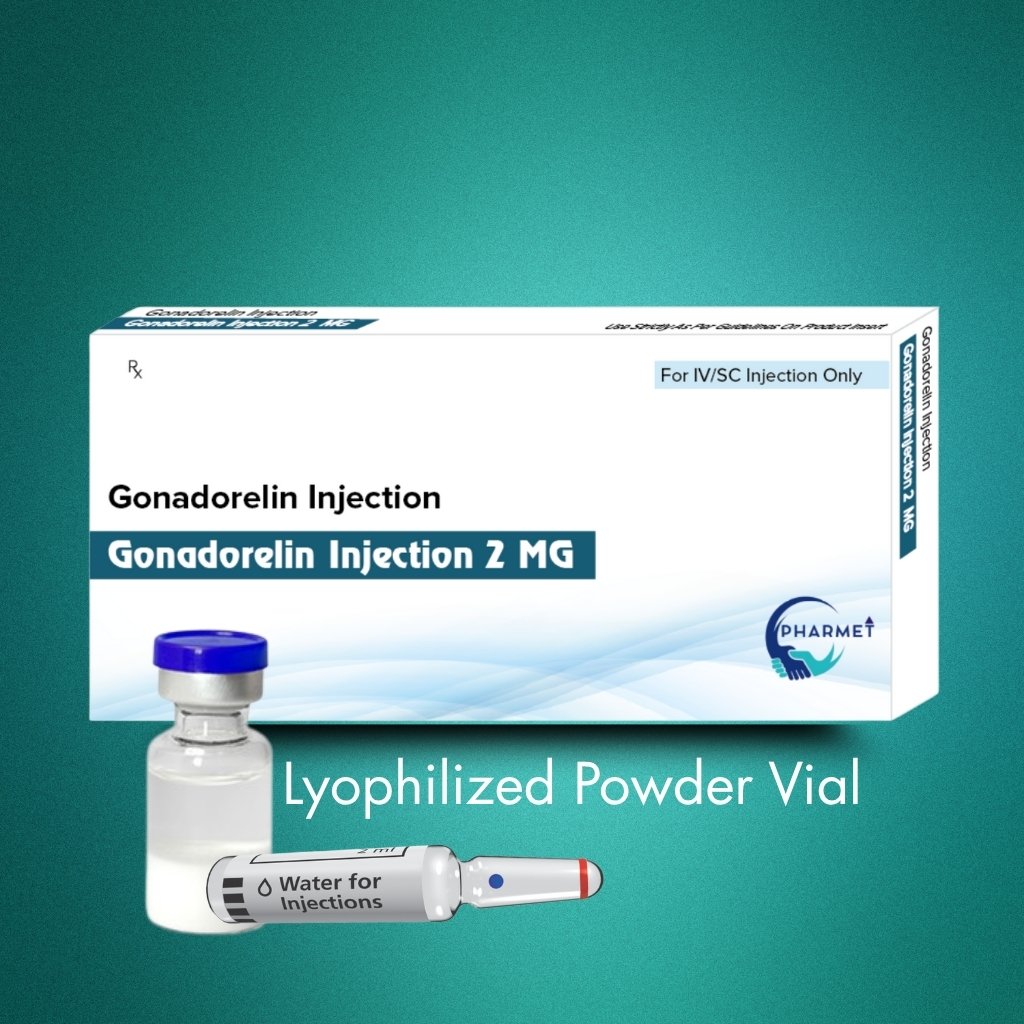
Gonadorelin Injection 2 MG is a synthetic form of the naturally occurring Gonadotropin-Releasing Hormone (GnRH), used primarily to assess and manage disorders of the hypothalamic-pituitary-gonadal axis. It plays a crucial role in stimulating the release of Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH), making it highly valuable in both diagnostic and therapeutic settings across endocrinology and infertility treatment.
This lyophilized formulation ensures stability, extended shelf life, and precision in dosing, supporting use in sensitive clinical applications under expert supervision.
Formulation & Presentation:
- Dosage Form: Injectable
- Presentation: Lyophilized Powder for Reconstitution
- Available Strengths: As per market requirement (commonly 2 MG)
Route of Administration:
- Injectable (Intravenous or Subcutaneous, as per clinical indication)
Therapeutic Indications:
- Diagnostic evaluation of pituitary function
- Hypogonadotropic hypogonadism
- Induction of ovulation in women with primary hypothalamic amenorrhea
- Stimulation of spermatogenesis in men
- Evaluation of delayed puberty
- Part of fertility treatment protocols in IVF centers
Side Effects:
- COMMON: Local irritation or redness at the injection site, Headache, Nausea, Flushing, Abdominal discomfort
- SERIOUS (RARE): Hypersensitivity reactions, Ovarian hyperstimulation (in rare fertility applications), Transient blood pressure fluctuations
- PRECAUTIONS: Should be administered under supervision of qualified healthcare professionals
- Use caution in patients with pre-existing cardiovascular or renal conditions
- Contraindicated in known hypersensitivity to GnRH or its analogues
- Not to be used during pregnancy unless clearly indicated
- Hormonal assessments and monitoring are essential during prolonged use
Be the first to review “GONADORELIN INJECTION 2 MG”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life: 24 months from date of manufacture or as per label specifications
- Storage: Store between 2°C to 8°C. Do not freeze. Protect from moisture and direct sunlight.
Regulatory Status & Supply Information:
- Regulatory Status: USFDA/WHO-GMP Compliant Manufacturing. Further certifications available on request
- Export-Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- IVF centers and fertility clinics requiring advanced hormonal therapies
- Endocrinologists and reproductive health specialists
- Distributors catering to gynecology, infertility, and hormone therapy portfolios
- Institutional buyers and tender authorities looking for high-purity hormone injections
- Hospitals and diagnostic labs involved in hormonal assay testing
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & flexible branding, packaging & formulation options
- End-to-end logistics flexibility & support
- Deep expertise in exporting specialty injectables & ART products
Contact Details:
- Email: hello@pharmet.io
- Phone/WhatsApp: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.