No products in the cart.
Description
PRODUCT DESCRIPTION:
Sodium Hyaluronate Injection is a sterile, viscoelastic solution derived through fermentation and purified to high molecular weight standards.
It mimics the natural hyaluronic acid found in synovial fluid and ocular tissues, making it a therapeutic lubricant and shock absorber in joints, or a space maintainer during ophthalmic surgery.
Formulated in ready-to-use Pre-Filled Syringes, this injectable gel is designed for precision delivery in osteoarthritis management, viscosupplementation, or as an adjuvant in ophthalmic procedures.
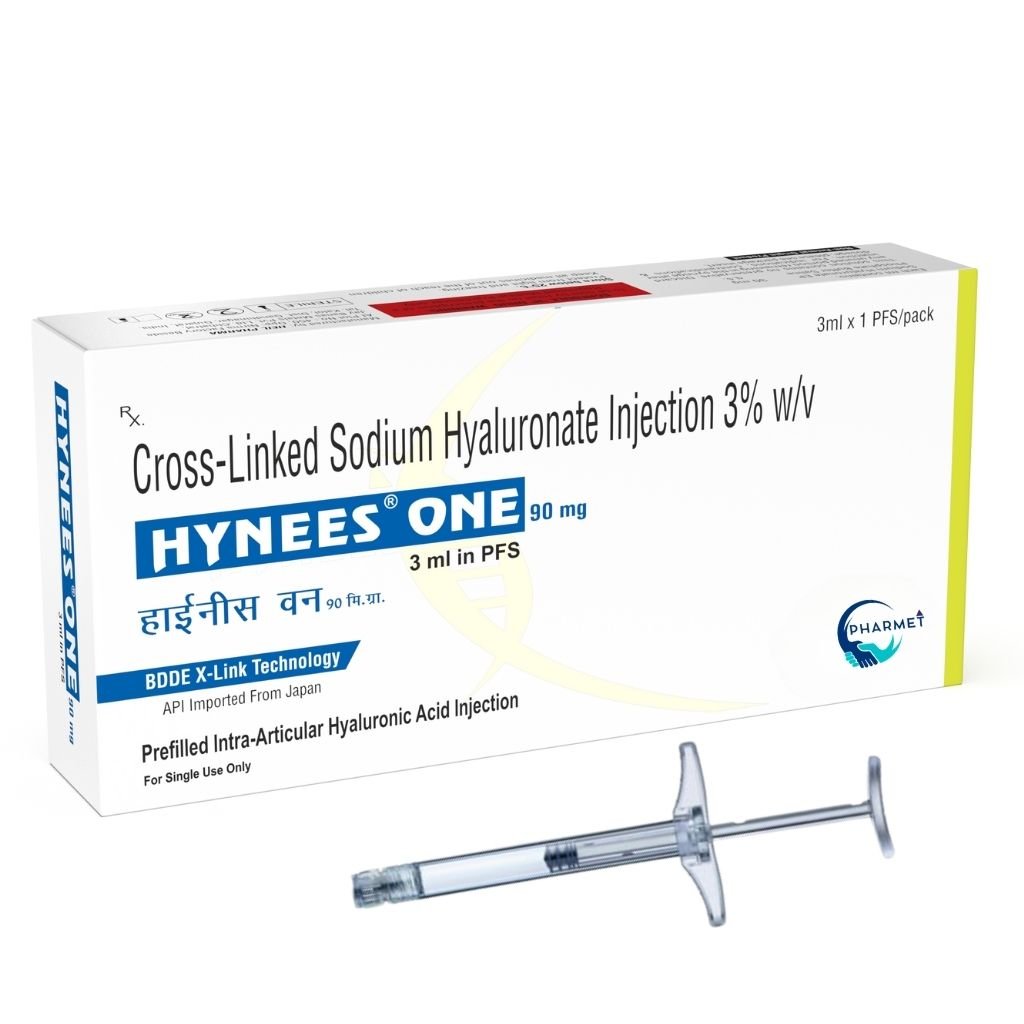
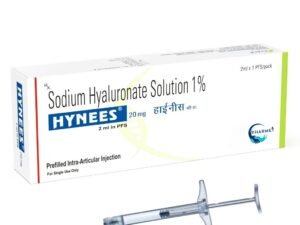
The higher concentration (3%) is specifically tailored for chronic knee osteoarthritis and deeper joint needs, while the 1% is optimal for moderate cases or ophthalmic applications.
Other Product Highlights: High-purity Sodium Hyaluronate/ Sterile, pyrogen-free, viscoelastic solution/ Complies with USP / EP standards/ Pre-Filled Syringe with luer-lock cap and plunger/ Verified by endotoxin, viscosity, and molecular weight tests
Formulation & Presentation:
- Dosage Form: Sterile Injectable (Sterile Lyophilized Solution for Reconstitution)
- Presentation: Pre-Filled Syringe (customizable as per market demand)
- Available Strengths:
- 20 MG/ 2 ML in 1% (Pre-Filled Syringe)
- 90 MG/ 3 ML in 3% (Pre-Filled Syringe)
- Route of Administration: Intra-Articular Use Only. To be used only in advanced clinical settings, as per clinical indication and severity. To be administered under supervision of a qualified specialist only. (As per physician’s discretion or decision)
Therapeutic Indications:
- Viscosupplementation for osteoarthritis of the knee and other synovial joints
- Relief from joint pain, stiffness, and limited mobility
- In ophthalmic surgery as a viscoelastic agent during procedures such as cataract extraction, corneal transplant, and glaucoma surgery
Side Effects:
- COMMON:
- Mild pain or swelling at the injection site
- Temporary joint stiffness
- Skin rash or redness near injection site
- Transient increase in joint discomfort post-injection
- SERIOUS (RARE):
- Hypersensitivity or allergic reactions
- Septic arthritis (if aseptic precautions not followed)
- Joint infection or hemarthrosis
- Vision disturbances (in ophthalmic use, if improperly administered)
- PRECAUTIONS:
- Strict aseptic technique must be followed during intra-articular or ophthalmic administration
- Not indicated for use in infected or inflamed joints
- Avoid use in patients with known allergy to avian proteins or hyaluronic acid derivatives
- Use caution in patients with pre-existing coagulopathies
- For professional medical use only
Be the first to review “Cross Linked Sodium Hyaluronate Injection”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards & import jurisdiction
- Weight: Approx. 40 GM per Carton (With Packaging)
- Dimensions: (15x1x8 CMS)
- Shelf Life (Subject To Country Specific Guidelines): 24 to 36 months under recommended storage conditions from date of manufacture or as per label specifications
- Storage: Store between 2°C to 25°C for Pre-Filled Syringes/Lyophilized (Do not freeze). Does not require cold-chain logistics. Protect from moisture and direct sunlight. The solution should be used strictly as per label guidelines.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. COA, MSDS, Stability Data, Manufacturing Batch Record, Dossier Support (on request). Further certifications available on request
- Exports Ready Dossiers: COA, COPP, Dossier Available (CTD/eCTD formats). Complies with USP/EP/BP Standards
- Stability: Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Orthopedic centers & sports injury clinics managing degenerative joint diseases
- Ophthalmic hospitals & eye surgery units requiring sterile viscoelastic agents
- Rehabilitation centers offering viscosupplementation therapies
- Government health missions & NGOs addressing arthritis and mobility care
- Importers and distributors in LATAM, Africa, CIS, and Southeast Asia supplying orthopedic and surgical-grade injectables
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & all products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.