No products in the cart.
Description
PRODUCT DESCRIPTION:
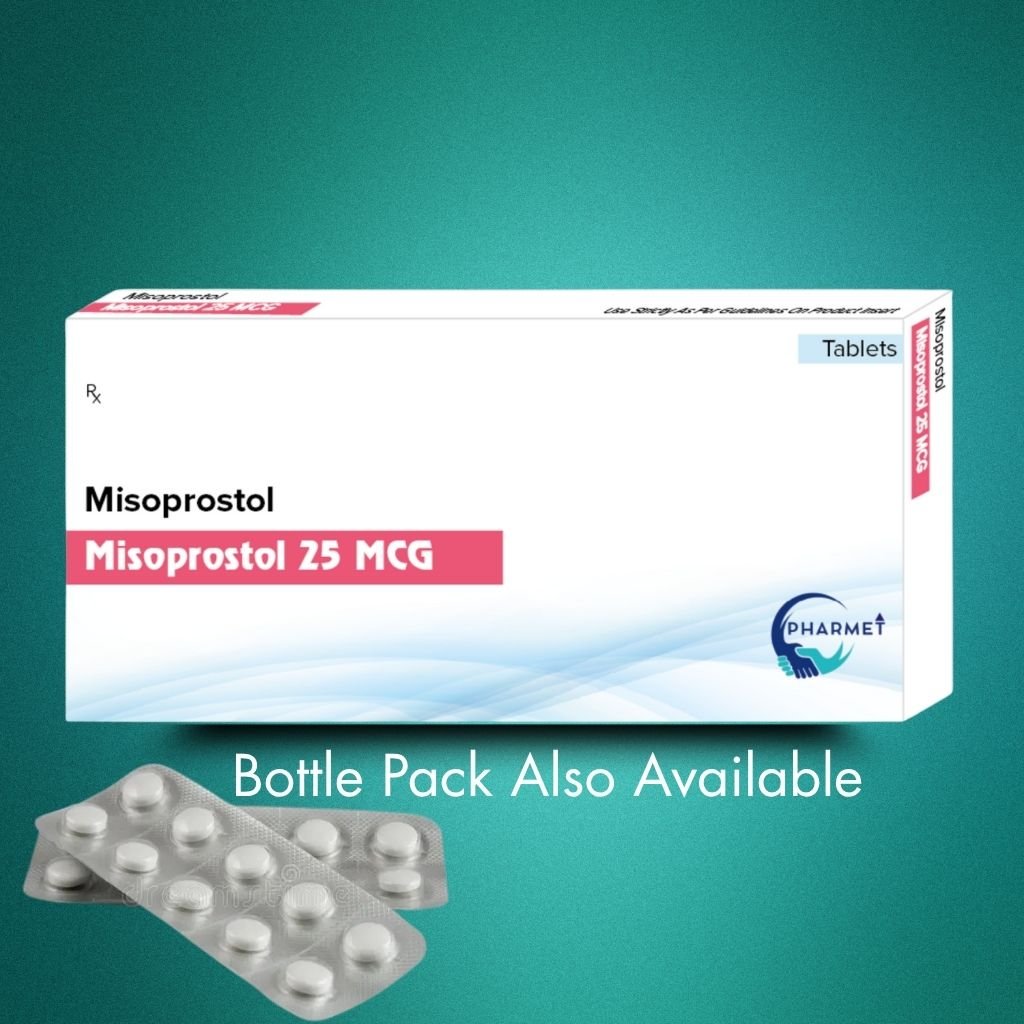
Misoprostol 25 MCG Tablets is a synthetic prostaglandin E1 (PGE1) analog used primarily for labor induction and cervical ripening in obstetric care. It stimulates uterine contractions and is also employed in the prevention of NSAID-induced gastric ulcers.
This low-dose variant is ideal for controlled obstetric use, offering precision and safety in administration.
Manufactured under WHO-GMP and stringent quality control protocols, this product is suited for government tenders, maternal health programs, and ethical markets.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths:
- Misoprostol 25 MCG Tablets
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Induction of labor at term or near-term
- Cervical ripening prior to surgical procedures
- Management of incomplete or missed abortion
- Gastric ulcer prevention in NSAID therapy (higher doses typically used)
Side Effects:
- COMMON: Uterine cramps, Nausea, Diarrhea, Headache, Chills or fever
- SERIOUS (RARE): Uterine rupture (especially in women with previous cesarean section), Excessive uterine contractions, Postpartum hemorrhage, Hypersensitivity reactions
- PRECAUTIONS:
- To be used under medical supervision only
- Contraindicated in women with prior uterine surgery or scar unless clearly indicated
- Monitor fetal heart rate and uterine activity during use
- Not to be used in pregnancy unless for medically indicated labor induction or abortion
Be the first to review “MISOPROSTOL TABLETS”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture and direct sunlight.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Government and maternal health programs focused on safe childbirth practices
- Hospitals and obstetric care providers requiring labor induction tools
- NGOs and humanitarian organizations focused on maternal and reproductive health
- Pharmaceutical distributors specializing in reproductive medicine
- Ethical procurement partners seeking low-dose uterotonic agents with global compliance
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support in
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io




Reviews
There are no reviews yet.