No products in the cart.
Description
PRODUCT DESCRIPTION:
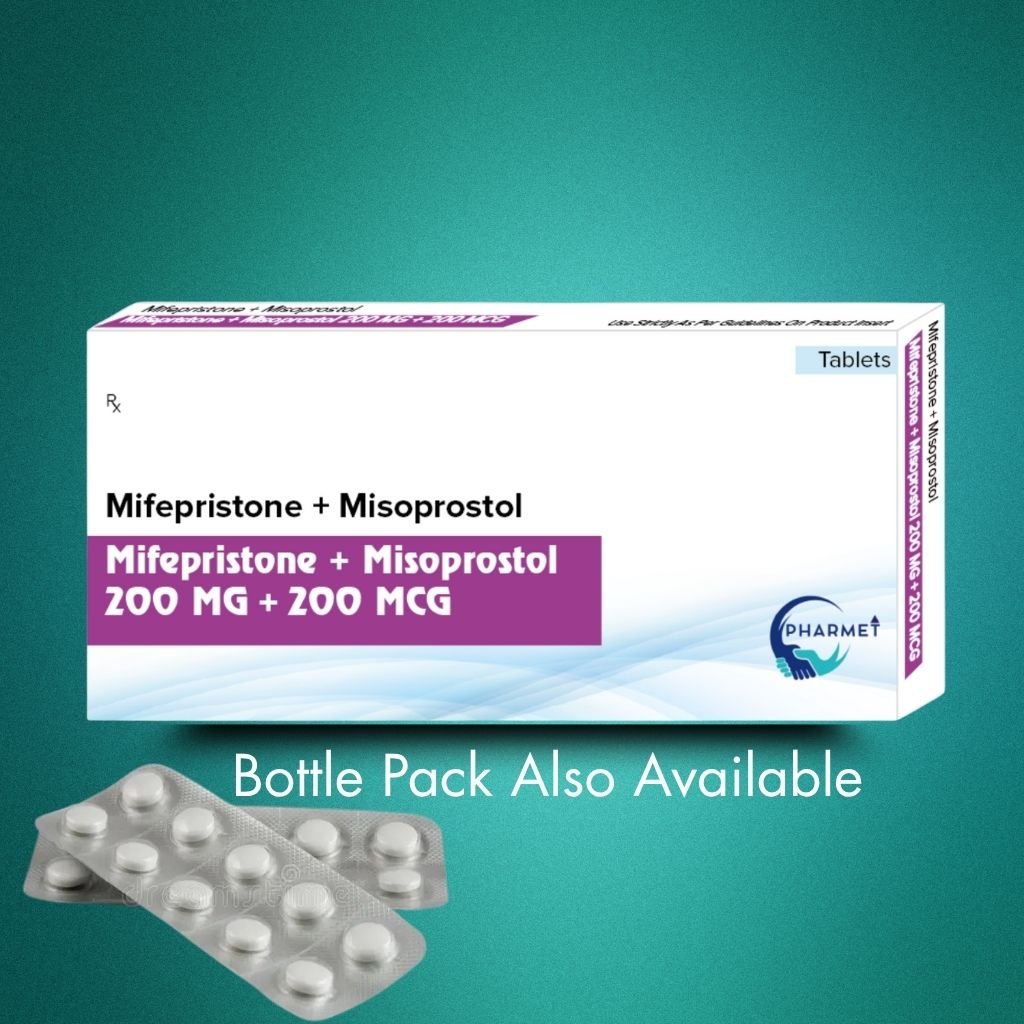
Mifepristone 200 MG + Misoprostol 200 MCG Tablets is a clinically approved combination used for medical termination of intrauterine pregnancy up to 63 days of gestation. Mifepristone, an anti-progestin, blocks the hormone necessary for pregnancy maintenance, while Misoprostol, a prostaglandin analog, induces uterine contractions to expel the pregnancy tissue.
This combination provides a non-invasive, safe, and effective option for medical abortion under healthcare supervision. Manufactured under WHO-GMP certified facilities, the product is designed for global compliance, ethical distribution, and maternal health programs.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Route of Administration: Buccal / Sublingual / Vaginal (Misoprostol as per indication)
- Available Strengths:
- Mifepristone 200 MG + Misoprostol 200 MCG Tablets
Therapeutic Indications:
- Medical termination of early pregnancy (≤ 63 days of gestation)
- Management of missed abortion
- Cervical preparation prior to surgical abortion
Side Effects:
- COMMON: Nausea, vomiting, Abdominal cramps, Diarrhea, Vaginal bleeding
- SERIOUS (RARE): Incomplete abortion, Severe uterine bleeding, Infection or septic shock, Hypersensitivity reactions
- PRECAUTIONS:
- To be administered under medical supervision only
- Should not be used in suspected ectopic pregnancy
- Avoid in patients with chronic adrenal failure or long-term corticosteroid therapy
- Emergency care facilities should be accessible in case of complications
- Pregnancy and gestational age must be confirmed prior to use
Be the first to review “Mifepristone + Misoprostol Tablets”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 to 36 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture and direct sunlight.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Public health departments and national reproductive care programs
- NGOs and humanitarian agencies supporting women’s health and safe abortion access
- Hospitals and OB-GYN clinics offering medical abortion services
- Pharmaceutical procurement agencies aligned with WHO and national protocols
- Ethical importers seeking compliant, quality-assured reproductive health solutions
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support in
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io



Reviews
There are no reviews yet.