No products in the cart.
Description
PRODUCT DESCRIPTION:
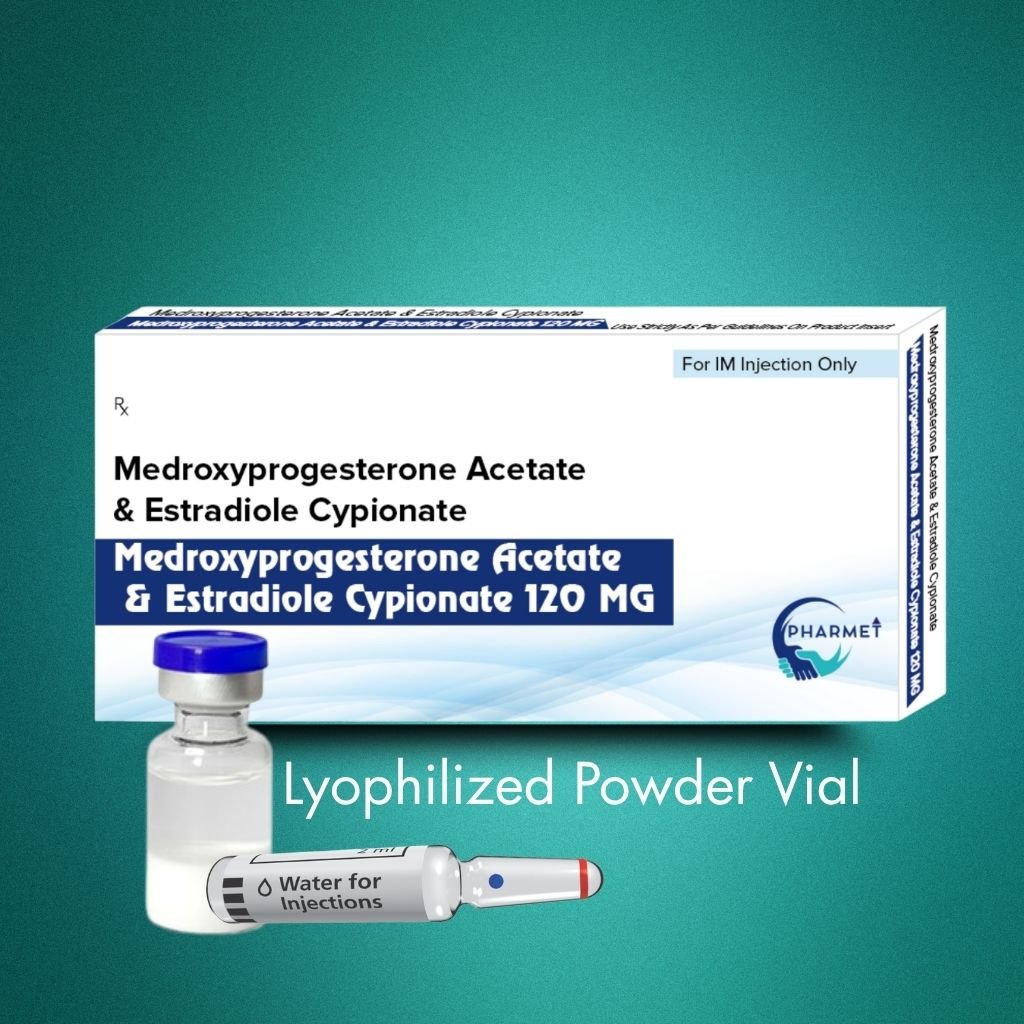
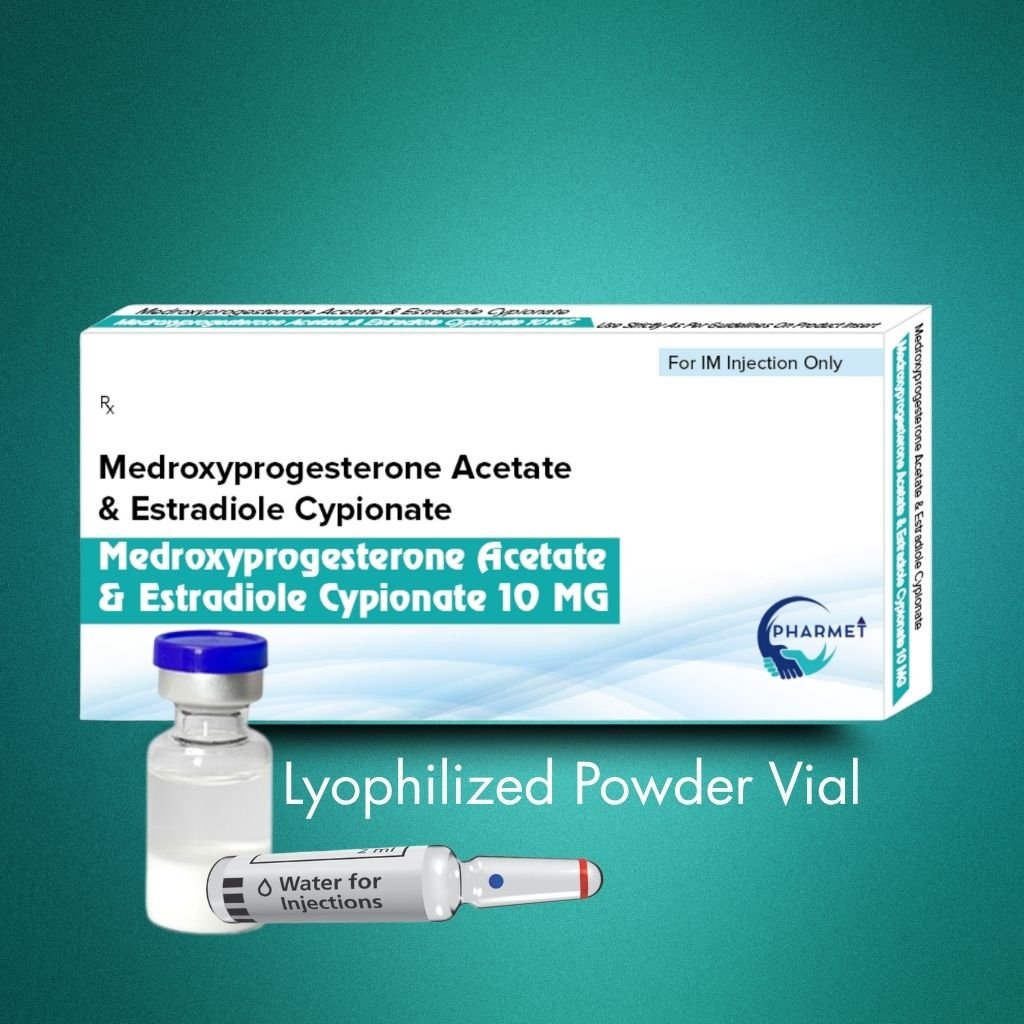
Medroxyprogesterone Acetate & Estradiol Cypionate Injection is a combined long-acting injectable contraceptive, offering extended protection through a synergistic blend of progestin and estrogen. Medroxyprogesterone Acetate provides potent ovulation suppression, while Estradiol Cypionate stabilizes the endometrium and improves menstrual cycle control.
Presented as a lyophilized powder with solvent, this formulation ensures stability, longer shelf life, and reconstitution accuracy, making it ideal for institutional, public health, and gynecology-based use. It is typically administered once every 28–30 days under medical supervision.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Lyophilized Powder With Solvent
- Available Strengths
- Medroxyprogesterone Acetate 120 MG
- Estradiol Cypionate 10 MG
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Long-term hormonal contraception
- Menstrual irregularities requiring hormonal regulation
- Adjunct therapy for women requiring estrogen-progestin support (under physician direction)
- Suitable for reproductive-age women seeking a non-daily contraceptive option
Side Effects:
- COMMON: Irregular menstrual bleeding or spotting, Headache, Breast tenderness, Nausea, Injection site reactions
- SERIOUS (RARE): Venous thromboembolism (VTE), Liver dysfunction, Mood changes or depressive symptoms, Bone mineral density reduction (with prolonged progestin use)
- PRECAUTIONS: Contraindicated during pregnancy or in women with undiagnosed vaginal bleeding
- Not suitable for women with a history of thromboembolic disorders or estrogen-sensitive cancers
- Long-term use should be accompanied by monitoring of bone health and liver function
- Should only be administered under medical supervision with appropriate screening
- Does not offer protection against HIV or other sexually transmitted infections
Be the first to review “MEDROXYPROGESTERONE ACETATE & ESTRADIOLE CYPIONATE”
TECHNICAL INFORMATION
Product Specifications
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life: 24 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Do not freeze. Protect from moisture and direct sunlight. Reconstituted solution: Use immediately after preparation or as per label
Regulatory Status & Supply Information
- Regulatory Status: WHO-GMP Compliant Manufacturing. Further certifications available on request
- Export-Ready Dossiers: Available in CTD/eCTD formats
- Stability: Zone IVB Compliant
- MOQ: Negotiable, based on market & registration status
- Export Options: Private label, institutional supply, or branded export
Who Should Source This Product?
- Public health programs offering long-acting contraceptive solutions
- Government and NGO-led reproductive health initiatives
- Hospitals and gynecology clinics offering once-a-month hormonal contraception
- Pharmaceutical distributors focused on women’s health and fertility care
- International tenders seeking combination hormone therapies with proven efficacy and safety
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration
- Trusted consortium network simplifying India's pharma supply chain
- Multiple approved manufacturers for best market fit
- Audited and compliant manufacturer network across India
- Flexible branding, packaging & formulation customization
- End-to-end logistics support
- Expertise in exporting specialty injectables & ART products
Contact Details
- Email: hello@pharmet.io
- Phone/WhatsApp: +91 9999091881
- Website: www.pharmet.io



Reviews
There are no reviews yet.