No products in the cart.
Description
PRODUCT DESCRIPTION:
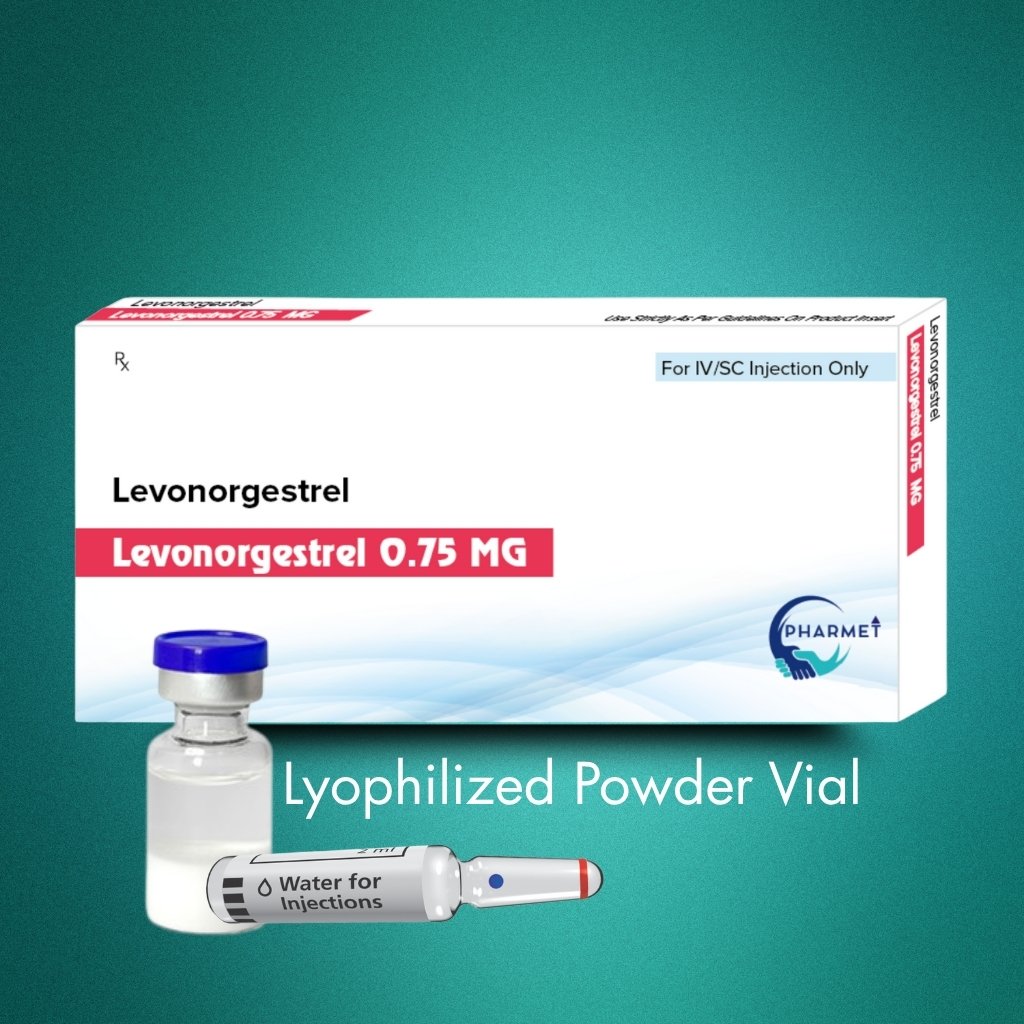
Levonorgestrel 0.75 MG Injection is a progestin-only emergency contraceptive designed for rapid, reliable prevention of unplanned pregnancies when administered within 72 hours of unprotected intercourse. Its injectable lyophilized formulation offers precise, effective hormone delivery in clinical settings where oral administration may be impractical or less reliable.
Used under medical supervision, this preparation ensures optimal bioavailability, faster onset of action, and is ideal for institutional or emergency contraceptive programs.
Formulation & Presentation:
- Dosage Form: Injectable
- Presentation: Lyophilized Powder for Reconstitution
- Available Strengths: As per market requirement (Commonly 0.75 MG)
- Route of Administration: Injectable (Intra-muscular or Subcutaneous, as per physician discretion)
Therapeutic Indications:
- Emergency contraception within 72 hours of unprotected sexual intercourse or contraceptive failure
- Hormonal modulation in specific gynecological protocols (off-label in select regimens)
Side Effects:
- COMMON Nausea and vomiting, Breast tenderness, Abdominal cramps, Fatigue, Headache, Menstrual irregularities
- SERIOUS (RARE): Allergic reactions, Severe abdominal pain suggestive of ectopic pregnancy (rare), Breakthrough bleeding requiring evaluation
- PRECAUTIONS: Should not be used as a regular contraceptive method
- Not effective once implantation has occurred
- Caution in women with a history of thromboembolic disorders, liver disease, or undiagnosed vaginal bleeding
- Does not protect against sexually transmitted infections (STIs)
- Medical supervision is advised before administration, especially in adolescents and women with comorbidities
Be the first to review “LEVONORGESTREL 0.75 MG INJECTION”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life: 24 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Do not freeze. Protect from moisture and direct sunlight. Reconstitute immediately before use.
Regulatory Status & Supply Information:
- Regulatory Status: USFDA/WHO-GMP Compliant Manufacturing. Further certifications available on request
- Export-Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Public health agencies and reproductive health programs offering emergency contraception
- NGOs and government bodies involved in maternal care and family planning
- Hospitals and clinics requiring emergency contraceptive stock for inpatient settings
- Pharmaceutical distributors focusing on gynecology and women’s health
- Buyers seeking cold-chain compatible, long-shelf-life hormonal injectables
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & flexible branding, packaging & formulation options
- End-to-end logistics flexibility & support
- Deep expertise in exporting specialty injectables & ART products
Contact Details:
- Email: hello@pharmet.io
- Phone/WhatsApp: +91 9999091881
- Website: www.pharmet.io



Reviews
There are no reviews yet.