No products in the cart.
Description
PRODUCT DESCRIPTION:
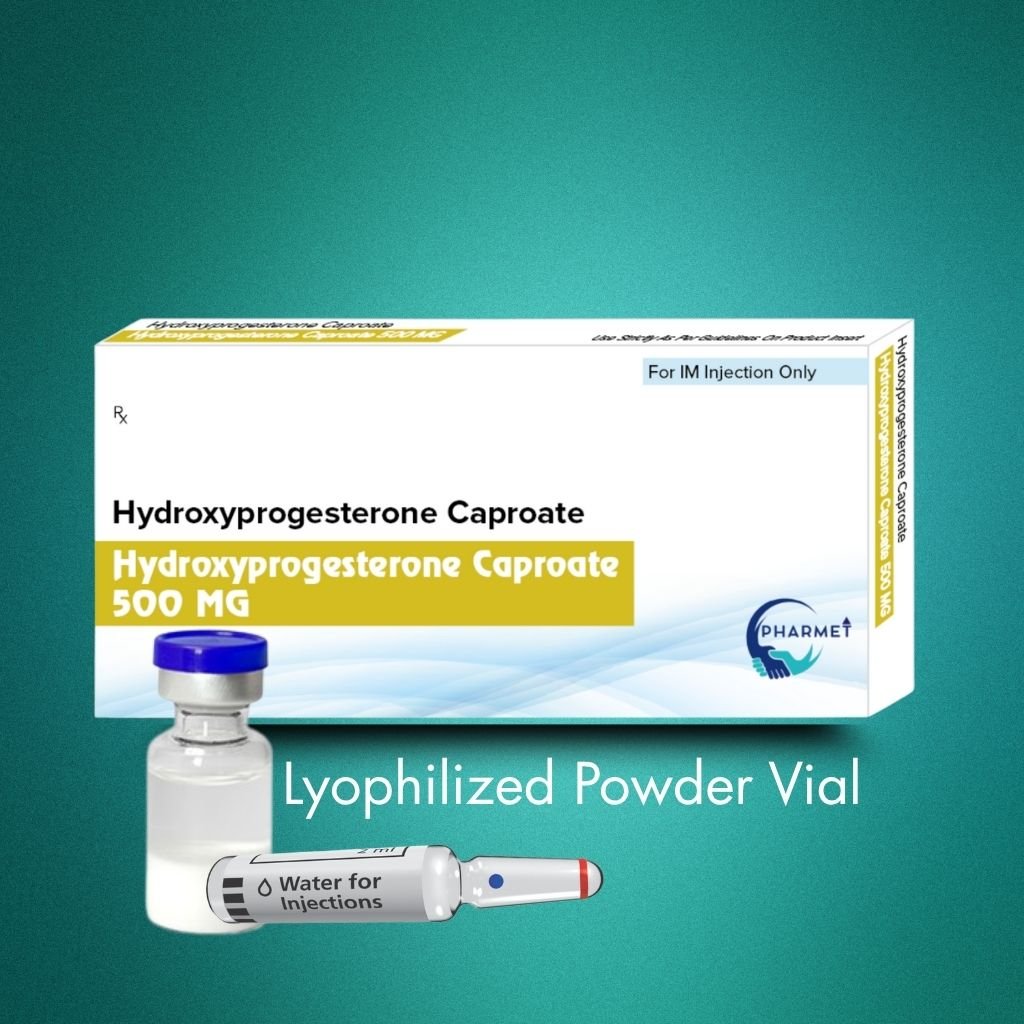
Hydroxyprogesterone Caproate Injection 500 MG is a long-acting progestin formulation widely used to reduce the risk of preterm birth in women with a history of spontaneous preterm delivery. Its high-dose, sustained-release profile ensures prolonged hormonal support during critical gestational windows.
It is also indicated in the treatment of threatened or habitual miscarriage, secondary amenorrhea, and other progesterone-responsive conditions. This formulation is available as a blister-packed ampoule or pre-filled syringe, designed for convenient administration in hospital and clinical settings.
Formulation & Presentation:
- Dosage Form: Injections
- Presentation: Blister Pack (Ampoules or Vials) Pre Filled Syringes also available
- Available Strengths: 500 MG Injections
- Route of Administration: IM (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Prevention of recurrent preterm birth in women with prior history
- Management of threatened and recurrent miscarriage
- Treatment of secondary amenorrhea
- Hormonal support in selected cases of infertility and assisted reproduction
- Management of certain endometrial disorders under clinical supervision
Side Effects:
- COMMON: Local pain, swelling, or irritation at injection site, Headache, Nausea, Fatigue or drowsiness, Breast tenderness
- SERIOUS (RARE): Allergic reactions (rash, itching, anaphylaxis), Fluid retention or swelling, Cholestatic jaundice, Depression or mood changes, Thromboembolic events (extremely rare with proper screening)
- PRECAUTIONS:
- Not indicated for use in early pregnancy without confirmed clinical need
- Contraindicated in patients with hormone-sensitive malignancies, active thromboembolic disorders, or liver disease
- Should be used cautiously in patients with a history of hypertension, diabetes, asthma, or migraine
- Periodic monitoring is recommended in long-term use
- Must be administered under the guidance of qualified healthcare professionals
Be the first to review “HYDROXYPROGESTERONE CAPROATE INJECTIONS”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture and direct sunlight. Do not refrigerate unless specified on the label.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Hospitals and maternity clinics focused on high-risk pregnancies
- Fertility and reproductive health centers
- Government maternal health programs and institutional tenders
- Distributors specializing in injectable hormonal therapies
- Importers targeting pregnancy care and gynecology portfolios
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support in
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.