No products in the cart.
Description
PRODUCT DESCRIPTION:
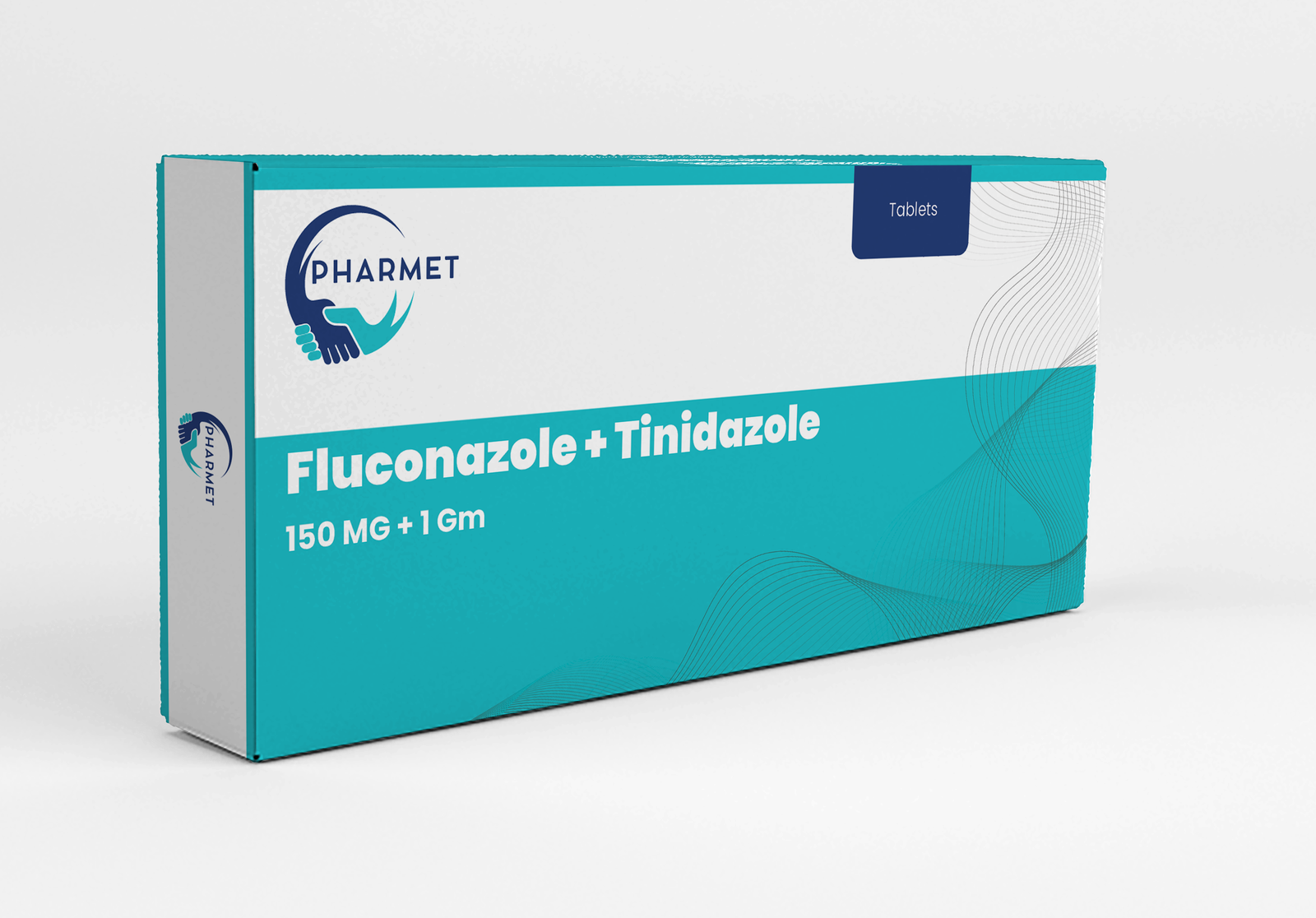
Fluconazole 150 MG + Tinidazole 1 GM Tablets combine the antifungal power of Fluconazole with the broad-spectrum antiprotozoal and antibacterial action of Tinidazole.
This fixed-dose combination is highly effective in treating mixed vaginal infections, including bacterial vaginosis, trichomoniasis, and candidiasis.
The synergistic action ensures rapid symptomatic relief and reduces recurrence by targeting both fungal and protozoal pathogens in a single dose regimen.
Ideal for mass health programs and institutional procurement where ease of compliance and broad infection coverage are critical.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths:
- Fluconazole: 150 MG + Tinidazole 1 GM Tablets
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Mixed vaginal infections (fungal + protozoal)
- Vaginitis due to Candida albicans and Trichomonas vaginalis
- Bacterial vaginosis
- Prevention of post-surgical or post-partum infections involving anaerobic flora
- Prophylactic use in high-risk gynecological settings
Side Effects:
- COMMON:
- Metallic taste
- Nausea
- Abdominal discomfort
- Headache
- Mild skin rash
- SERIOUS (RARE):
- Liver enzyme elevations
- Peripheral neuropathy (on prolonged use)
- Allergic skin reactions
- QT prolongation (with co-administered drugs)
- PRECAUTIONS:
- Avoid alcohol consumption during and 72 hours after Tinidazole use (disulfiram-like reaction)
- Use cautiously in hepatic impairment
- Monitor ECG if co-administered with QT-prolonging agents
- Not recommended during first trimester of pregnancy
- Ensure accurate diagnosis of mixed infections before use
Be the first to review “Fluconazole 150 MG + Tinidazole 1 GM Tablets”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: (15x1x8 CMS)
- Shelf Life (Subject To Country Specific Guidelines): 24 to 36 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture, direct sunlight & children.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Government health departments running reproductive and maternal health programs
- Hospitals and clinics managing high-volume outpatient gynecology
- NGOs and humanitarian groups offering STI and women’s health care
- Distributors seeking high-demand anti-infective combinations
- Importers supplying to emerging healthcare markets across LATAM, Africa & Asia
Why Source from PHARMET?
- Oncology-focused distributors in emerging and regulated markets
- Government tenders and hospital procurement departments
- Cancer foundations and treatment support NGOs
- Pharma wholesalers serving LATAM, Africa, Southeast Asia
- Regulatory-compliant buyers seeking dossier-backed hormone therapies
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.