No products in the cart.
Description
PRODUCT DESCRIPTION:
Favipiravir Tablets are broad-spectrum antiviral agents designed to inhibit viral RNA-dependent RNA polymerase, a key enzyme responsible for viral replication.
Originally developed for resistant influenza strains, Favipiravir has shown promise in treating a range of RNA viruses, including in emergency use during outbreaks such as COVID-19.
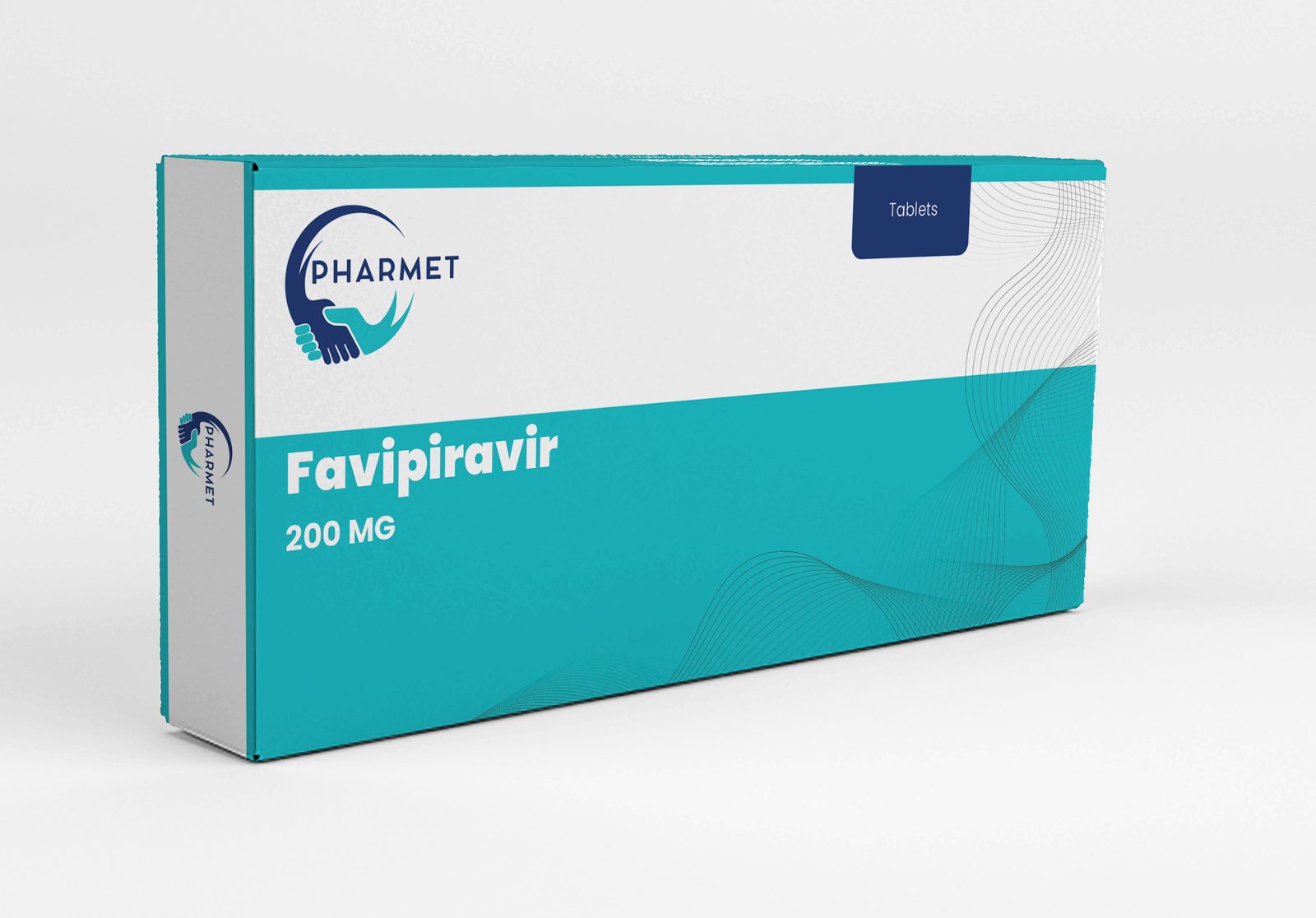
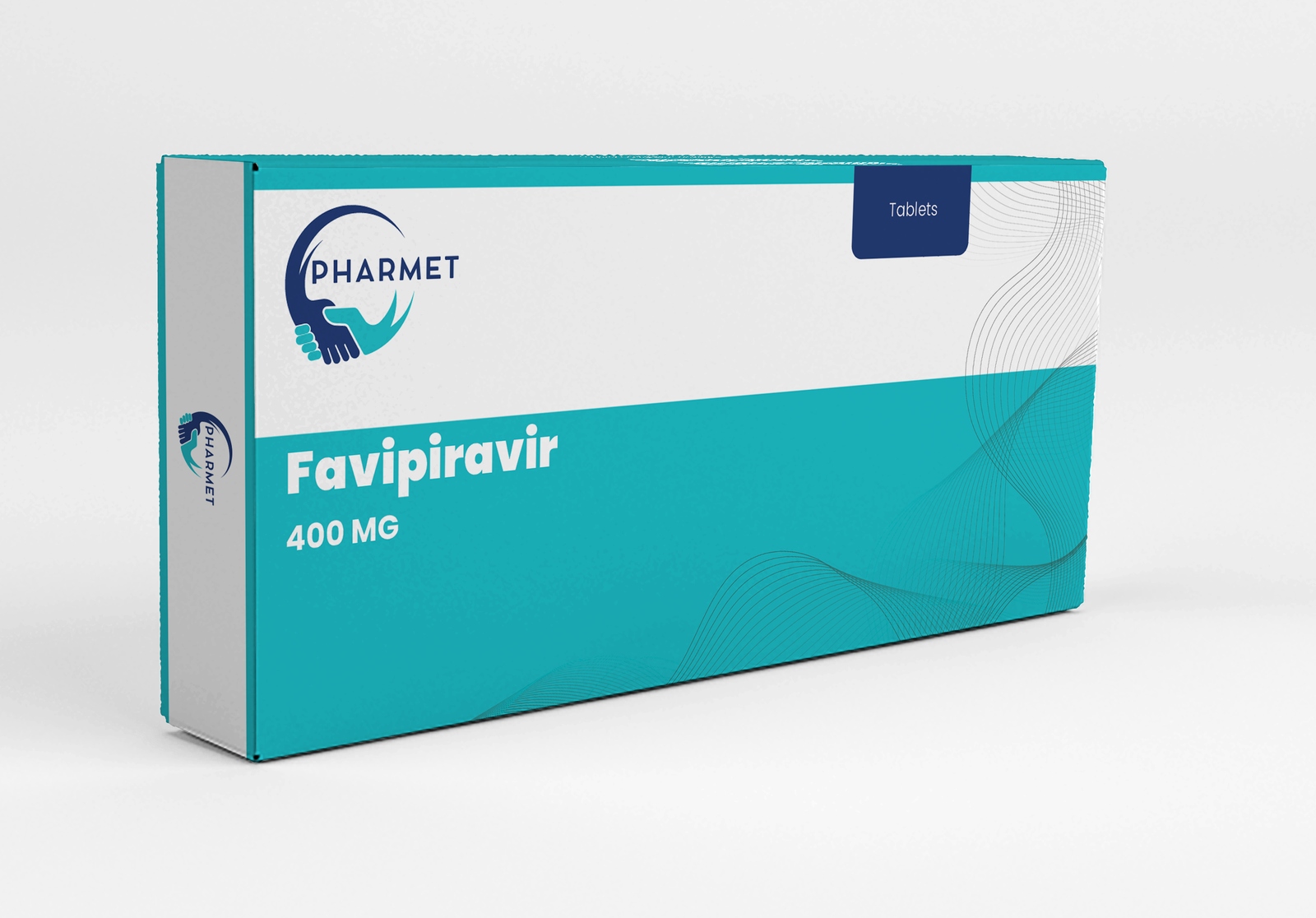
Offered in strengths of 200 MG and 400 MG, these tablets are especially valuable for stockpiling in public health emergencies, pandemic preparedness programs, and hospital protocols for severe viral respiratory infections.
Manufactured under strict regulatory compliance, Favipiravir is trusted by governments and global health organizations for rapid deployment when speed and efficacy matter most.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths:
- Favipiravir Tablets: 200 MG
- Favipiravir Tablets: 400 MG
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Treatment of mild to moderate influenza in adults
- Emergency use in respiratory RNA virus infections, including COVID-19 (as per national protocols)
- Considered for use during viral outbreaks under regulatory approvals
Side Effects:
- COMMON:
- Elevated liver enzymes
- Diarrhea
- Decreased neutrophil count
- Increased uric acid levels
- Gastrointestinal discomfort
- SERIOUS (RARE):
- Teratogenicity (contraindicated in pregnancy)
- QT interval prolongation (cardiac monitoring advised in at-risk patients)
- Acute hypersensitivity reactions
- Hepatic toxicity (in prolonged use)
- PRECAUTIONS:
- Strictly contraindicated in pregnancy and lactation
- Liver function and uric acid levels must be monitored during treatment
- Not recommended in patients with severe hepatic or renal impairment
- Use only under supervision in emergency protocols or approved national programs
Avoid concomitant use with known QT-prolonging agents
Be the first to review “Favipiravir Tablets, 200 MG, 400 MG”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: (15x1x8 CMS)
- Shelf Life (Subject To Country Specific Guidelines): 24 to 36 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture, direct sunlight & children.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Government agencies maintaining emergency antiviral stockpiles
- Hospitals and ICUs managing viral respiratory illness protocols
- Global health organizations responding to epidemic/pandemic outbreaks
- NGO-led field medical units in conflict or outbreak regions
- Ethical distributors supplying essential emergency-use medications
Why Source from PHARMET?
- Oncology-focused distributors in emerging and regulated markets
- Government tenders and hospital procurement departments
- Cancer foundations and treatment support NGOs
- Pharma wholesalers serving LATAM, Africa, Southeast Asia
- Regulatory-compliant buyers seeking dossier-backed hormone therapies
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io

Reviews
There are no reviews yet.