No products in the cart.
Description
PRODUCT DESCRIPTION:
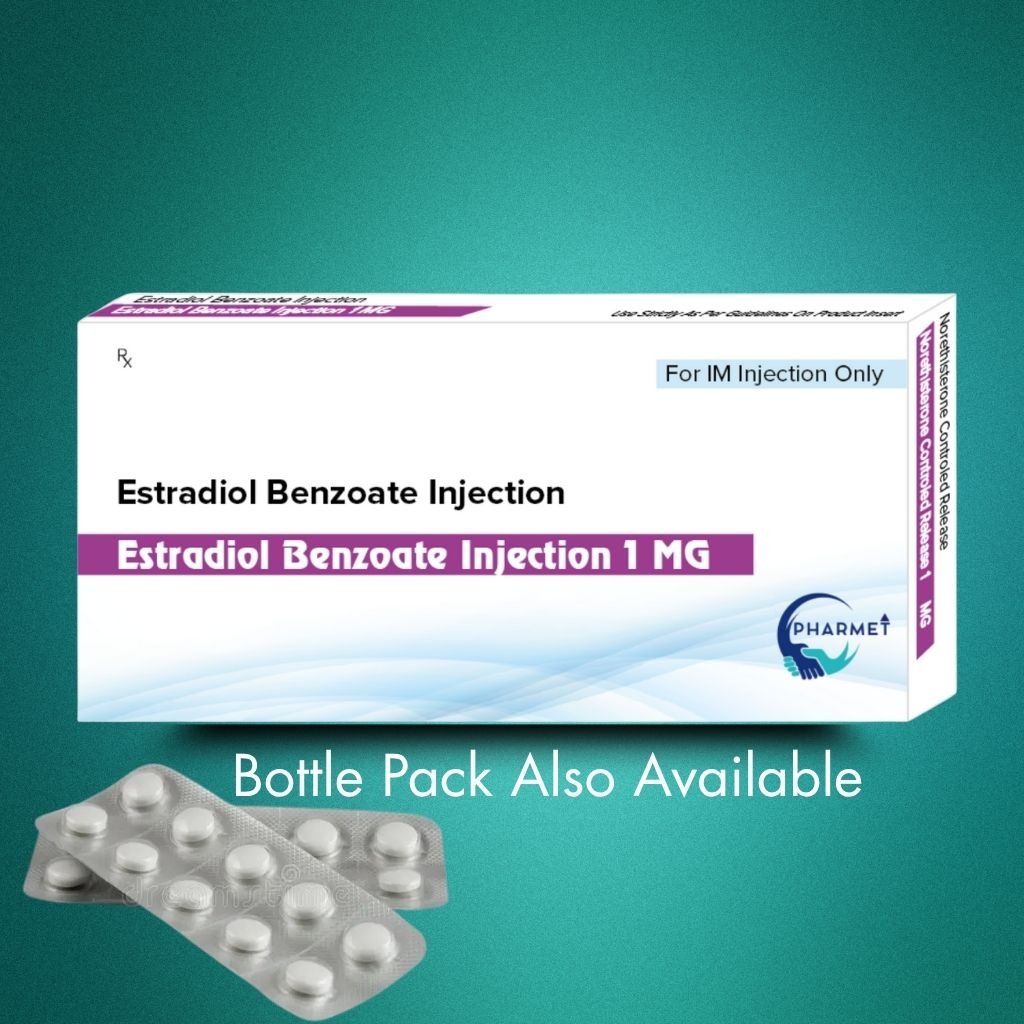
Estradiol Benzoate Injection is a long-acting estrogen preparation used widely in hormone replacement therapy (HRT), menstrual irregularities, and infertility treatments. As a potent estrogenic compound, it plays a key role in regulating ovulation and supporting endometrial development in women.
It is also used in transgender hormone therapy protocols and certain veterinary applications. Its injectable form ensures rapid and sustained release, offering clinical flexibility and improved patient compliance.
Formulation & Presentation:
- Dosage Form: Injectable Solution
- Presentation: Glass Vial / Ampoule
- Available Strengths: As per market requirement (commonly 1 mg/mL or 5 mg/mL)
Route of Administration:
- Injectable (As per clinical therapeutic indication and treatment protocol)
Therapeutic Indications:
- Hormone Replacement Therapy (HRT) in estrogen deficiency
- Menstrual irregularities such as amenorrhea
- Ovulation induction in infertility treatment
- Part of gender-affirming hormone therapy protocols
- Veterinary applications (in select geographies)
Side Effects:
- COMMON: Nausea, Breast tenderness, Fluid retention, Mood swings
- SERIOUS (RARE): Thromboembolic disorders, Gallbladder disease, Liver dysfunction, Hypertension
- PRECAUTIONS: Contraindicated in estrogen-dependent cancers, active thromboembolic disorders, and liver disease
- Use with caution in patients with cardiovascular risks or migraines
- Routine monitoring recommended during long-term therapy
Be the first to review “ESTRADIOL BENZOATE INJECTION”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture
- Storage: Store in a cool, dry place below 25°C. Protect from moisture and direct sunlight
Regulatory Status & Supply Information:
- Regulatory Status: USFDA/WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- IVF & Fertility Clinics
- Gynecology & Endocrinology Specialists
- LGBTQ+ Health Programs & Transgender Clinics
- Veterinary Health Distributors (where approved)
- Government & NGO Procurement Missions
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io




Reviews
There are no reviews yet.