No products in the cart.
Description
PRODUCT DESCRIPTION:
Entecavir Tablets are potent oral antiviral agents used for the treatment of chronic Hepatitis B virus (HBV) infection in adults and children above 2 years with active viral replication and evidence of liver inflammation.
As a guanosine nucleoside analogue, Entecavir inhibits HBV polymerase with high selectivity and potency, helping to reduce viral load and improve liver function over time.
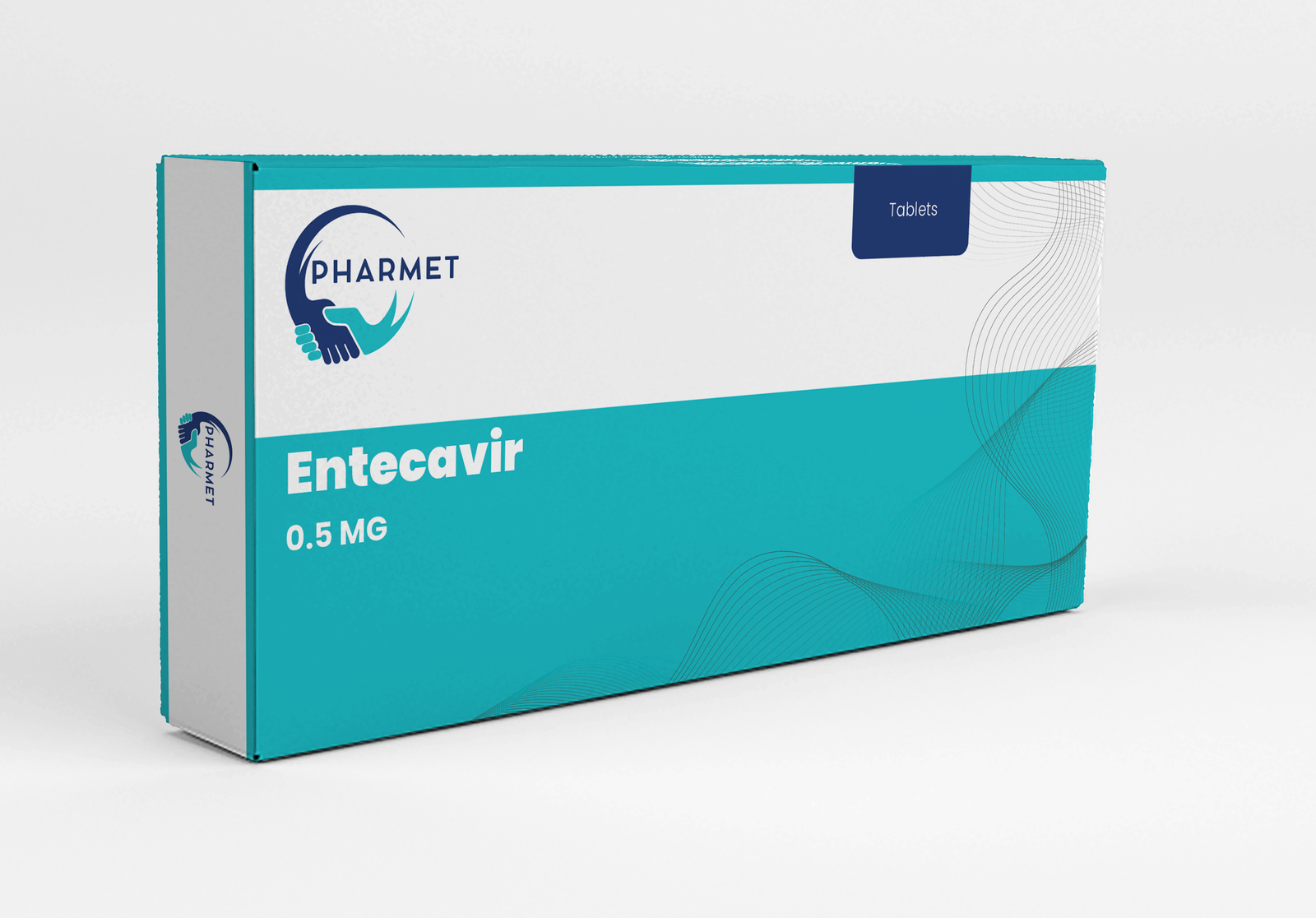
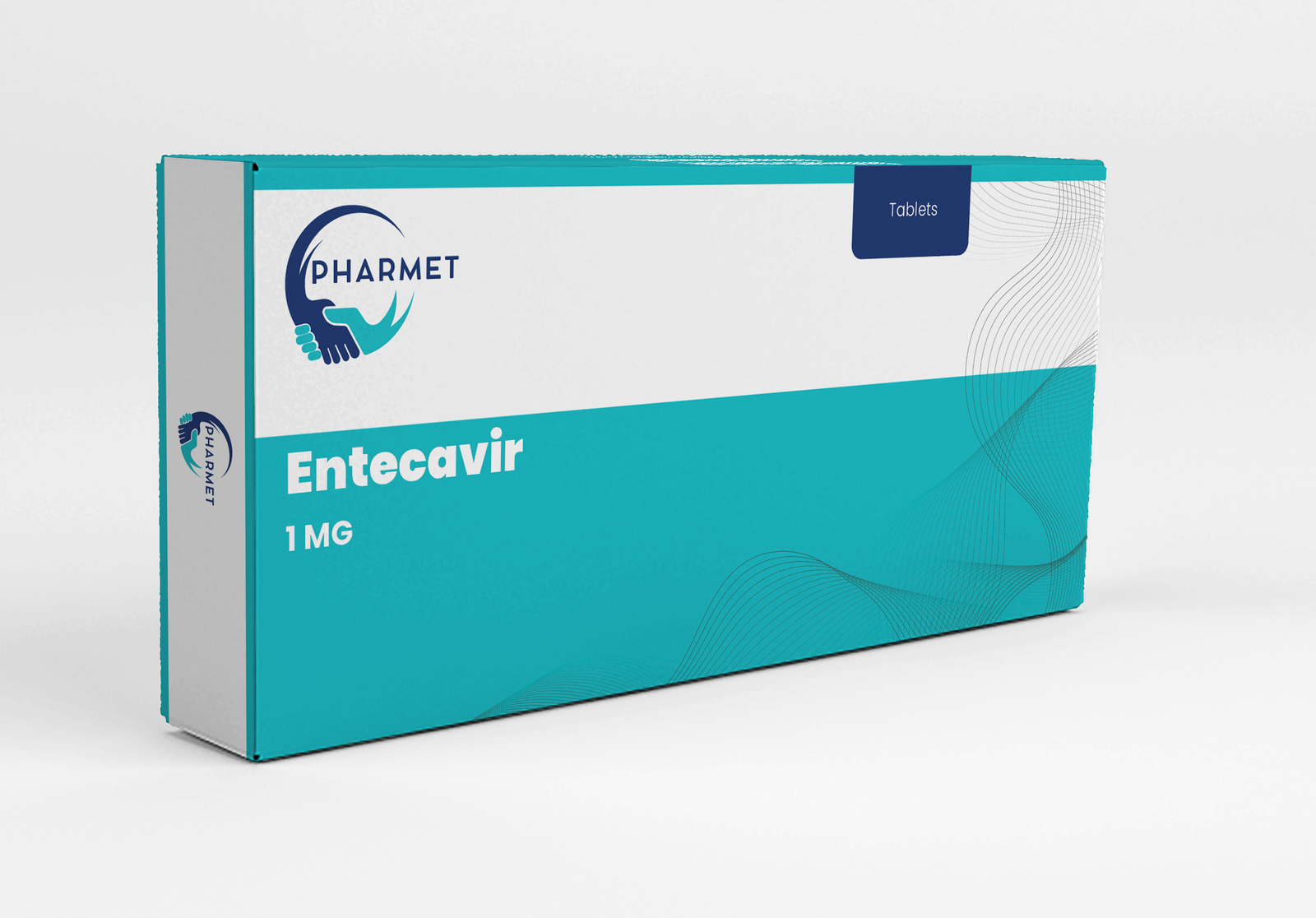
Available in strengths of 0.5 MG and 1 MG, Entecavir is an integral part of national hepatitis programs and long-term viral suppression protocols.
Produced under stringent GMP-compliant facilities, this product ensures therapeutic consistency, ideal for public health tenders and ethical procurement.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths:
- Entecavir Tablets: 0.5 MG
- Entecavir Tablets: 1 MG
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Chronic Hepatitis B infection in nucleoside-treatment-naive adults with evidence of active viral replication
- Hepatitis B treatment in patients with compensated or decompensated liver disease
- Pediatric use in HBV-positive children aged ≥2 years with liver inflammation
Side Effects:
- COMMON:
- Headache
- Fatigue
- Dizziness
- Nausea
- Diarrhea
- SERIOUS (RARE):
- Lactic acidosis
- Hepatomegaly with steatosis
- Exacerbation of hepatitis upon discontinuation
- Renal dysfunction in long-term therapy
- PRECAUTIONS:
- Monitor renal function during long-term therapy
- Regular liver function tests are recommended
- Not recommended during pregnancy unless clearly needed
- Discontinuation may cause rebound hepatitis flare—monitor closely
- Dose adjustment required in renal impairment
Be the first to review “Entecavir Tablets, 0.5 MG, 1 MG”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: (15x1x8 CMS)
- Shelf Life (Subject To Country Specific Guidelines): 24 to 36 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture, direct sunlight & children.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Public health agencies targeting Hepatitis B elimination
- NGOs engaged in viral hepatitis screening and treatment programs
- Government supply chains managing liver disease therapies
- Hospitals and specialty clinics in hepatology and infectious diseases
- Ethical procurement agencies supplying essential antiviral medicines
Why Source from PHARMET?
- Oncology-focused distributors in emerging and regulated markets
- Government tenders and hospital procurement departments
- Cancer foundations and treatment support NGOs
- Pharma wholesalers serving LATAM, Africa, Southeast Asia
- Regulatory-compliant buyers seeking dossier-backed hormone therapies
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.