No products in the cart.
Description
PRODUCT DESCRIPTION:
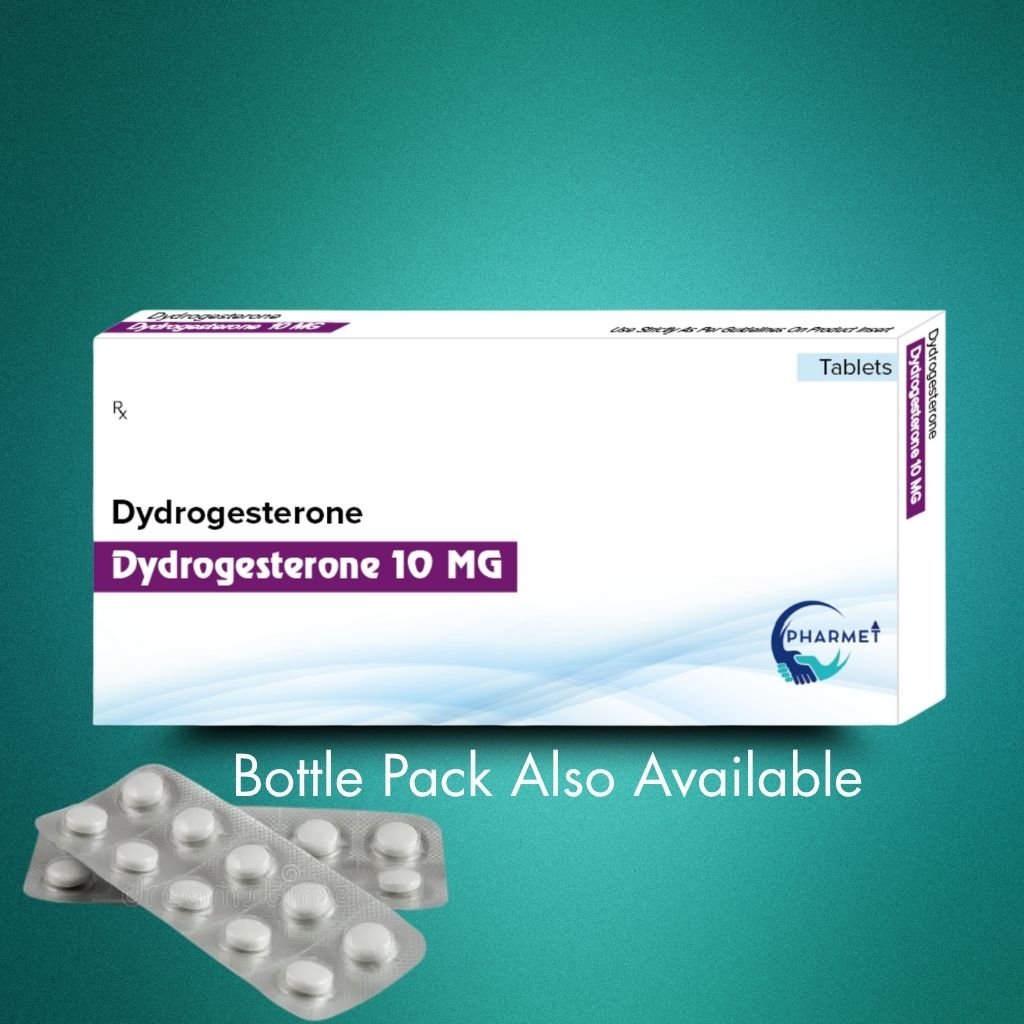
Dydrogesterone 10 MG Tablets are a synthetic oral progestogen designed to closely mimic the activity of natural progesterone. With its high selectivity and excellent oral bioavailability, Dydrogesterone plays a crucial role in treating a variety of gynecological disorders, particularly those linked to progesterone deficiency.
It is especially indicated for use in luteal phase support, infertility treatment, menstrual irregularities, and hormone replacement therapy (HRT). Dydrogesterone is well-tolerated, non-androgenic, and does not inhibit ovulation—making it ideal for use in fertility management.
Formulation & Presentation:
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths: Dydrogesterone 10 MG Tablet
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Luteal phase support in IVF and other Assisted Reproductive Technologies (ART)
- Progesterone deficiency-related infertility
- Secondary amenorrhea
- Dysmenorrhea (painful periods)
- Irregular menstrual cycles
- Threatened or recurrent miscarriage
- Endometriosis
- Hormone Replacement Therapy (in combination with estrogens)
Side Effects:
- COMMON: Nausea, Headache, Breast tenderness, Bloating, Drowsiness or fatigue
- SERIOUS (RARE): Hypersensitivity reactions, Jaundice or liver dysfunction, Breakthrough bleeding, Mood disturbances
- PRECAUTIONS: Not to be used in known or suspected hormone-sensitive malignancies
- Caution in patients with liver dysfunction, undiagnosed vaginal bleeding, or a history of thromboembolism
- Should be used under medical supervision, especially during pregnancy
- Regular monitoring advised when used for long-term HRT or fertility protocols
Be the first to review “DYDROGESTERONE”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture or as per label specifications
- Storage: Store below 25°C. Protect from moisture and direct sunlight.
Regulatory Status & Supply Information:
- Regulatory Status: WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- IVF clinics and fertility centers
- Gynecology and reproductive endocrinology practices
- Distributors catering to hormonal therapies and women’s health
- Tender authorities sourcing for ART, HRT, and maternity health programs
- Importers focused on high-demand women’s health and menstrual health segments
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support in
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io





Reviews
There are no reviews yet.