No products in the cart.
Description
PRODUCT DESCRIPTION:
Cetrorelix Acetate is a synthetic gonadotropin-releasing hormone (GnRH) antagonist used to prevent premature luteinizing hormone (LH) surges in women undergoing controlled ovarian stimulation for assisted reproductive technologies (ART), including IVF.
Unlike GnRH agonists, Cetrorelix provides immediate suppression of LH without an initial hormonal flare, allowing for better cycle control and reduced risk of ovarian hyperstimulation syndrome (OHSS).
Formulation & Presentation:
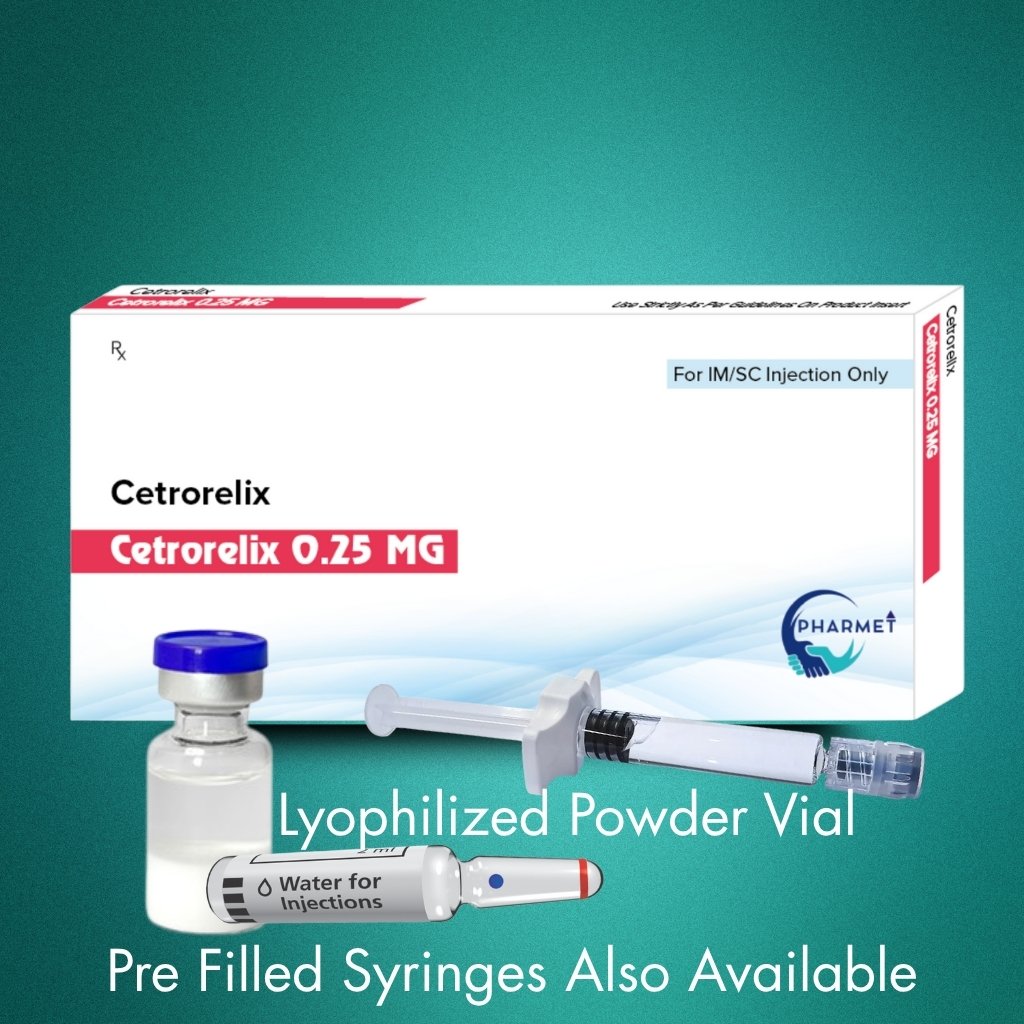
- Dosage Form: Lyophilized Powder for Reconstitution
- Presentation: Sterile Vial/ Pre-Filled Syringes (PFS) Also Available
- Available Strengths: 0.25 MG Injection
Route of Administration:
- Subcutaneous (As per indication & Specialist Directions)
Therapeutic Indications:
- Prevention of premature LH surges in women undergoing controlled ovarian stimulation for ART
- IVF and embryo transfer cycle management
Side Effects:
- COMMON: Mild abdominal pain, injection site redness or itching, headache, nausea, fatigue.
- SERIOUS (RARE): Ovarian hyperstimulation syndrome (OHSS), hypersensitivity reactions, shortness of breath, hives.
- PRECAUTIONS: Should be administered under expert medical supervision.
- Monitoring of ovarian response and hormone levels is critical.
- Use with caution in patients with known hypersensitivity to GnRH analogues
Be the first to review “CETRORELIX ACETATE INJECTION”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: 300439
- Weight: Approx. 40g per vial (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture
- Storage: Store strictly between 2°C to 8°C. Protect from light. Do not freeze.
Regulatory Status & Supply Information:
- Regulatory Status: USFDA/WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
Who Should Source This Product?
- Fertility clinics and ART centers
- National ART Programs or reproductive health programs
- Distributors of hormone-based injectables or Women’s reproductive Health programs
- NGOs and procurement agencies in reproductive care focusing in infertility treatment
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io



Reviews
There are no reviews yet.