No products in the cart.
Description
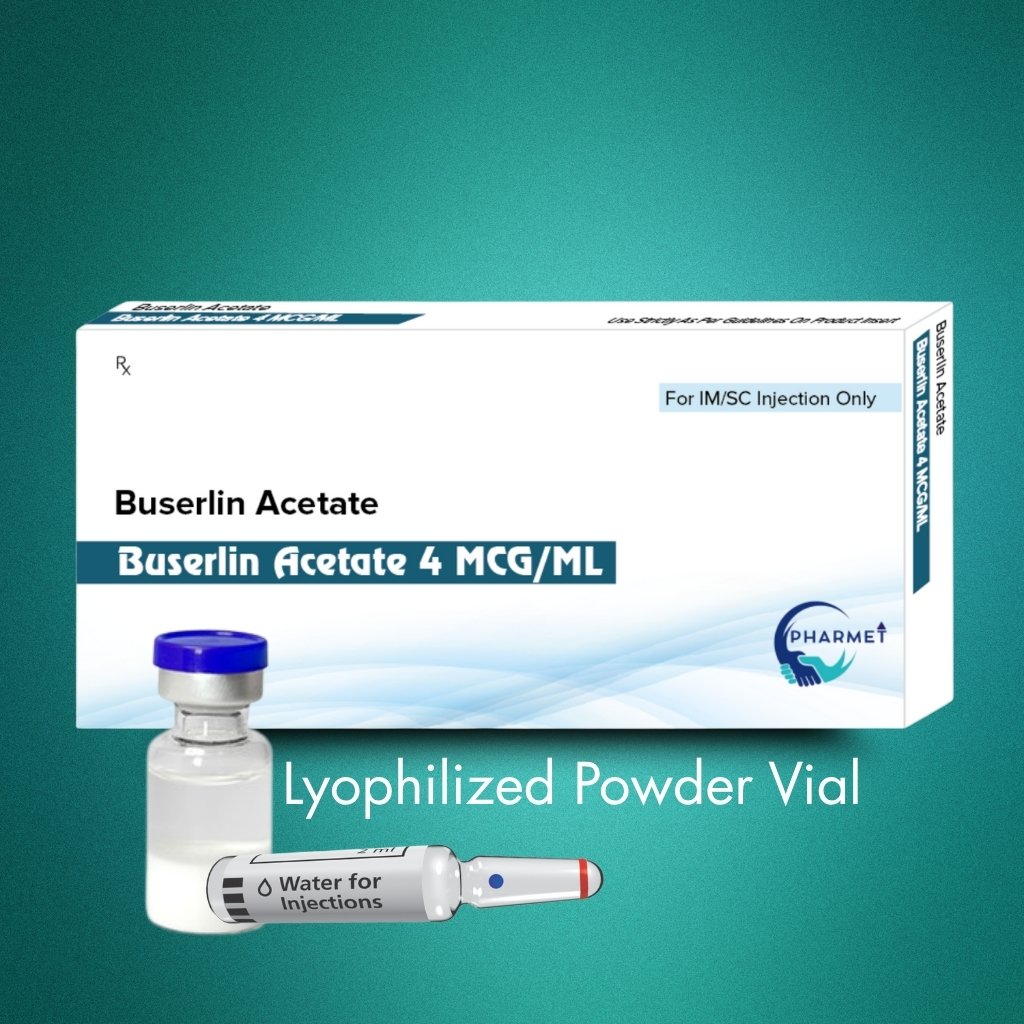
PRODUCT DESCRIPTION:
Buserelin Acetate is a synthetic analogue of the naturally occurring gonadotropin-releasing hormone (GnRH), used primarily to regulate hormonal secretion through receptor desensitization.
It is widely used in reproductive medicine, gynecology, and oncology to suppress gonadotropin production, particularly in conditions requiring temporary suppression of estrogen or testosterone levels. Its potent action makes it essential in assisted reproductive protocols and hormone-dependent cancers.
Formulation & Presentation:
- Dosage Form: Lyophilized Powder for Reconstitution
- Presentation: Sterile Vial/ Pre-Filled Syringes (PFS) Also Available
- Available Strengths: 4 MCG Injection
Route of Administration:
- Intramuscular / Sub-Cutaneous (as per indication and clinical protocol)
Therapeutic Indications:
- Hormone-sensitive prostate cancer
- Endometriosis
- Uterine fibroids
- Assisted Reproductive Techniques (e.g., IVF protocols for pituitary downregulation)
- Precocious puberty (as per specialist direction)
Side Effects:
- COMMON: Hot flashes, headache, decreased libido, mood changes, sweating, injection site irritation
- SERIOUS (RARE): Osteoporosis (with prolonged use), visual disturbances, cardiovascular symptoms, allergic reactions
- PRECAUTIONS: Long-term use may lead to bone density loss—consider concurrent bone protection measures
- Use with caution in patients with cardiovascular or psychiatric history
- Therapy should be initiated and monitored by specialists experienced in hormone therapy
Be the first to review “BUSERLIN ACETATE INJECTION”
TECHNICAL INFORMATION
Product Specifications:
- HSN Code: 300439
- Weight: Approx. 40g per vial (With Packaging)
- Dimensions: 15x1x8 CMS
- Shelf Life (Subject To Country Specific Guidelines): 24 months from date of manufacture
- Storage: Store strictly between 2°C to 8°C. Protect from light. Do not freeze.
Regulatory Status & Supply Information:
- Regulatory Status: USFDA/WHO-GMP Complaint Manufacturing. Further certifications available on request
- Exports Ready Dossiers: Dossier Available (CTD/eCTD formats)
- Stability: Zone IVB Compliant
- MOQ: Negotiable. Based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Fertility clinics and reproductive endocrinology centers
- Oncology distributors handling hormone-sensitive cancers
- Hospitals and health departments managing hormone therapy protocols
- Buyers in need of long-acting GnRH agonists for regulated markets
- Distributors of hormone-based injectables or Women’s reproductive Health programs
- NGOs and procurement agencies in hormonal treatment
Why Source from PHARMET?
- Single-window sourcing for diverse pharma needs
- Export-ready documentation & regulatory support
- Adaptable documentation support for effective registration support
- Trusted consortium network, simplifying India’s complex pharma supply chain
- Multiple approved manufacturers for best fit in import country
- Network of audited, compliant manufacturers across India
- Customizable & Flexible branding, packaging & formulation flexibility
- End to End Logistics support flexibility & support
- Deep expertise in exporting specialty injectables & ART products
CONTACT DETAILS:
- E-MAIL: hello@pharmet.io
- Phone/WhatsApp Number: +91 9999091881
- Website: www.pharmet.io


Reviews
There are no reviews yet.