No products in the cart.
Description
PRODUCT DESCRIPTION:
Artemether + Lumefantrine is a highly effective, fixed-dose artemisinin-based combination therapy (ACT) used as the gold standard in the treatment of uncomplicated Plasmodium falciparum malaria. This synergistic pairing brings together two potent antimalarial agents:
- Artemether, a fast-acting artemisinin derivative, rapidly kills malaria parasites during the blood stage of infection, delivering quick symptomatic relief within 24–48 hours.
- Lumefantrine, with a longer half-life, eliminates residual parasites and prevents recurrence by ensuring sustained antimalarial activity even after Artemether has been metabolized.
This combination is endorsed by the World Health Organization (WHO) and is widely deployed in malaria-endemic regions, including sub-Saharan Africa, Southeast Asia, and parts of South America.
It is suitable for adults and pediatric populations and is included in national malaria treatment guidelines across many countries.
The tablets are film-coated, stable in tropical conditions, and formulated for optimal bioavailability when taken with food. Their blister-packed presentation allows for extended shelf life and easy field distribution, making them ideal for public health campaigns, NGO supplies, and institutional tenders.
Formulation & Presentation:
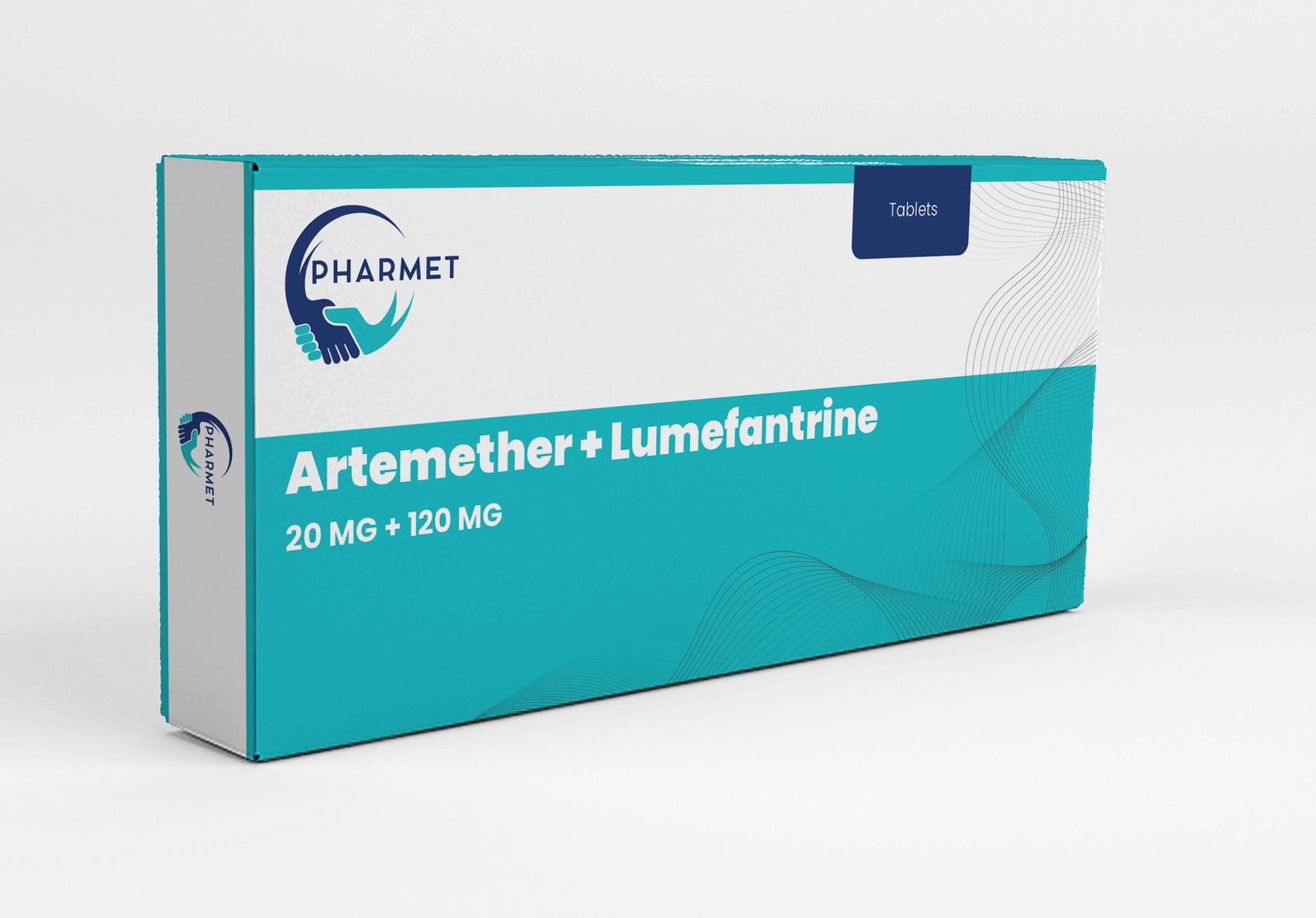
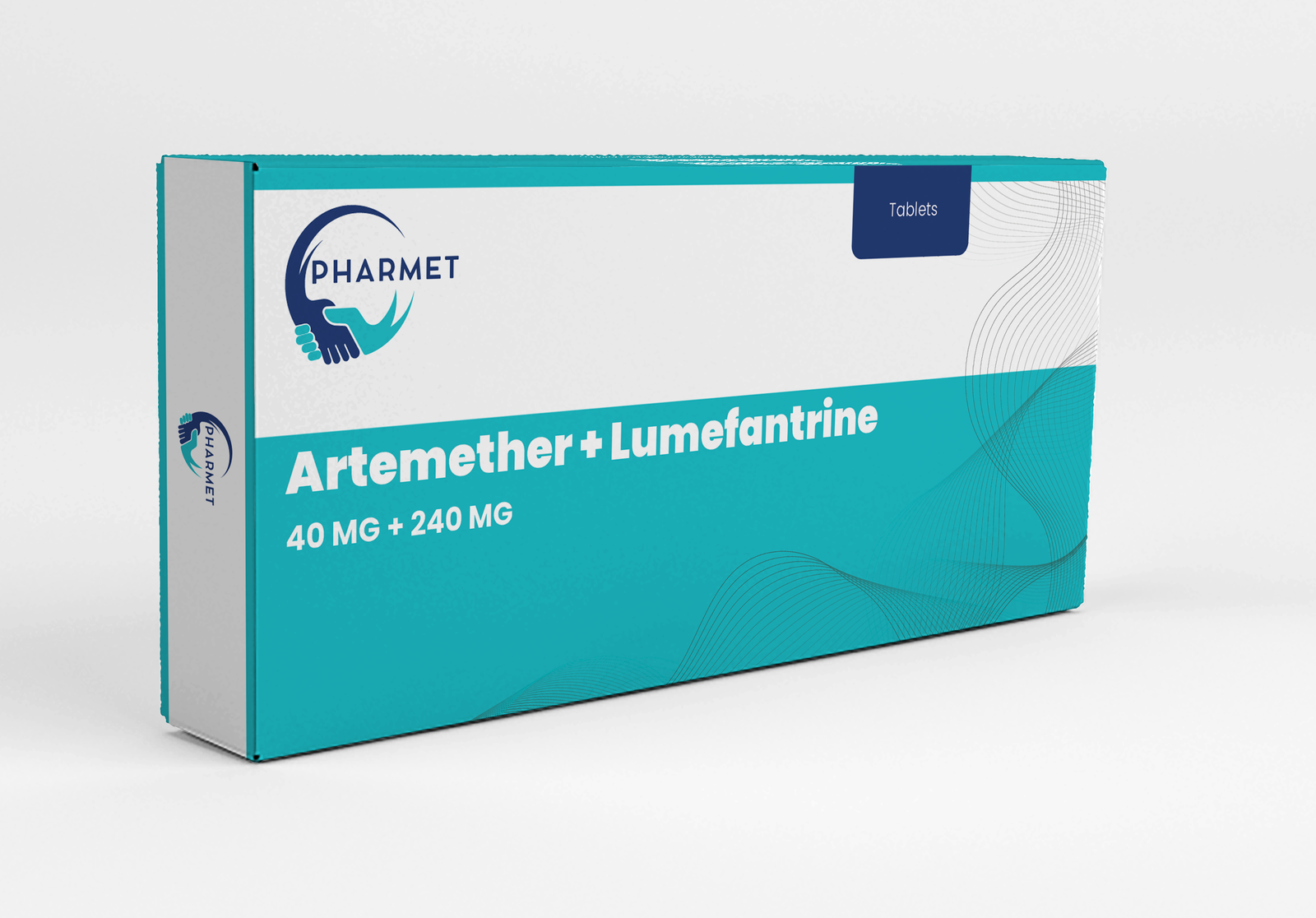
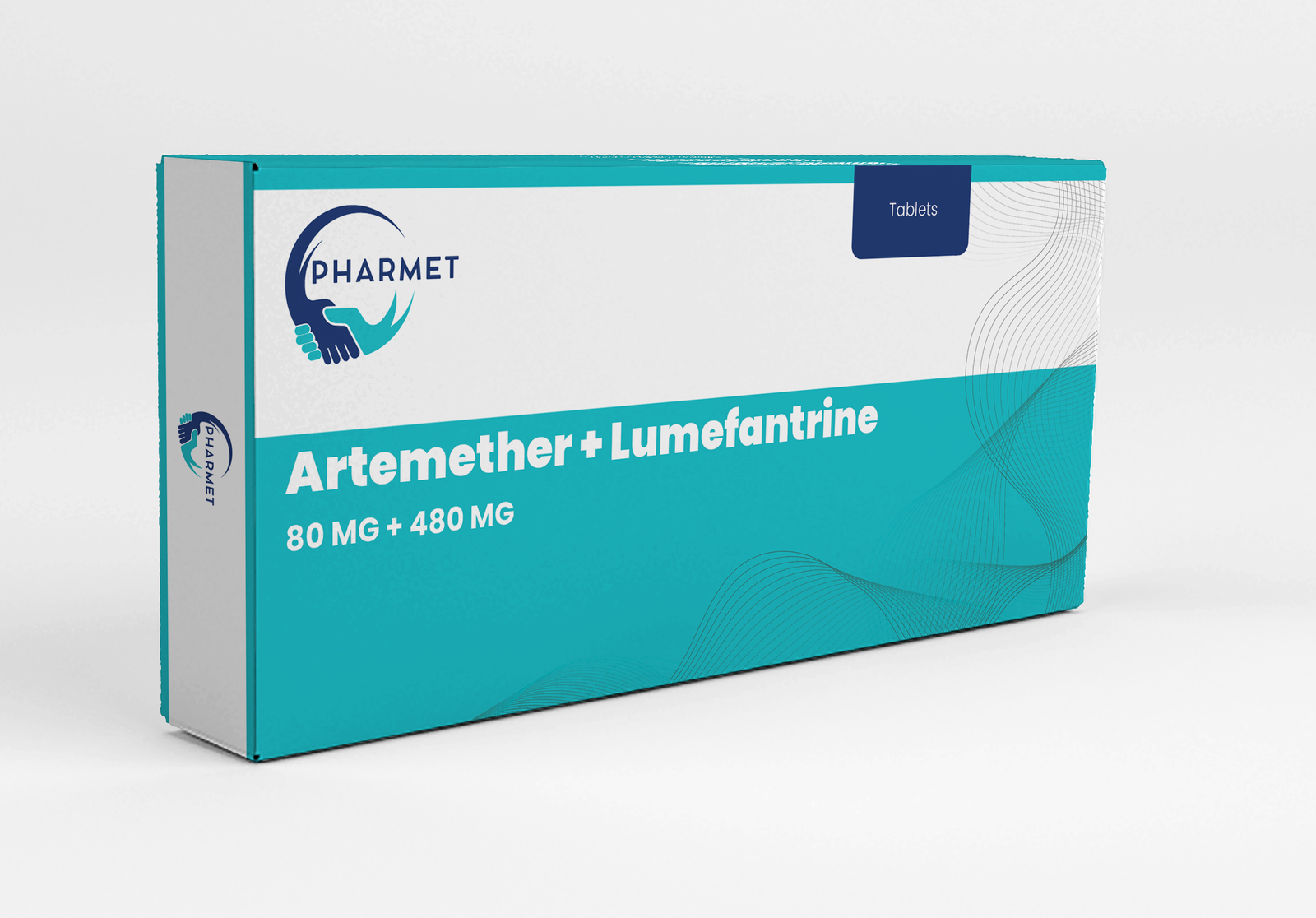
- Dosage Form: Tablets
- Presentation: Available in Blister Pack or HDPE Bottle (As per market requirement)
- Available Strengths:
- Artemether 20 MG + Lumefantrine: 120 MG Tablets
- Artemether 40 MG + Lumefantrine: 240 MG Tablets
- Artemether 80 MG + Lumefantrine: 480 MG Tablets
- Route of Administration: Oral (As per physician’s discretion or therapeutic indication)
Therapeutic Indications:
- Treatment of uncomplicated falciparum malaria
- Effective against multi-drug-resistant strains
- Recommended as first-line ACT therapy in most malaria control programs
Side Effects:
- COMMON:
- Headache
- Dizziness
- Nausea
- Anorexia
- Abdominal pain
- Fatigue
- SERIOUS (RARE):
- QT prolongation
- Hypersensitivity reactions
- Hepatotoxicity
- Palpitations, arrhythmias or syncope
- PRECAUTIONS:
- Use with caution in patients with cardiac conditions or QT prolongation
- Avoid in early pregnancy unless absolutely necessary
- Monitor liver function with prolonged use
- Take with food (preferably high fat) to enhance absorption
Be the first to review “Artemether + Lumefantrine Tablets 20 MG + 120 MG, 40 MG + 240 MG, 80 MG + 480 MG”
TECHNICAL INFORMATION
Product Specifications
- HSN Code: To be assigned per international classification standards
- Weight: Approx. 40g per Carton (With Packaging)
- Dimensions: 15 x 1 x 8 CMS
- Shelf Life: 24–36 months from date of manufacture (subject to country-specific guidelines or label specifications)
- Storage: Store below 25°C to 30°C. Protect from moisture and direct sunlight.
Regulatory Status & Supply Information
- Regulatory Status: WHO-GMP Compliant Manufacturing. Further certifications available on request
- Export-Ready Dossiers: Available in CTD/eCTD formats
- Stability: Zone IVB Compliant
- MOQ: Negotiable based on market & registration status
- Export Options: Available for private label, institutional supply, or branded export
Who Should Source This Product?
- Public Health Departments and National Malaria Control Programs
- Government procurement bodies and Ministries of Health
- NGOs and International Health Agencies working in malaria-endemic zones
- Hospitals, Community Clinics, and Global Health Distributors
Why Source from PHARMET?
- Oncology-focused distributors in emerging and regulated markets
- Government tenders and hospital procurement departments
- Cancer foundations and treatment support NGOs
- Pharma wholesalers serving LATAM, Africa, Southeast Asia
- Regulatory-compliant buyers seeking dossier-backed hormone therapies
Contact Details
- Email: hello@pharmet.io
- Phone/WhatsApp: +91 9999091881
- Website: www.pharmet.io




Reviews
There are no reviews yet.